special offers
View all
Special Offer
£29
£25
excl VAT
Special Offer
Add to FavoritesRemove from Favourites
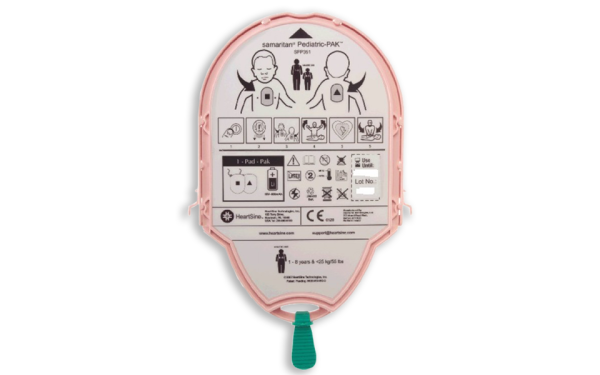
Heartsine
Samaritan Pad-Pak (<8yrs or <25kg) Combined Battery & Electrode Cartridge
Add to Favorites
£165
£150
excl VAT
Special Offer
£26
£21
excl VAT
Special Offer
£ 70.00 – £200.00
£60.00 – £190.00
excl VATpopular products
View all
Subscription
£5.50
£4.50
/ month
excl VAT
Subscription
Add to FavoritesRemove from Favourites
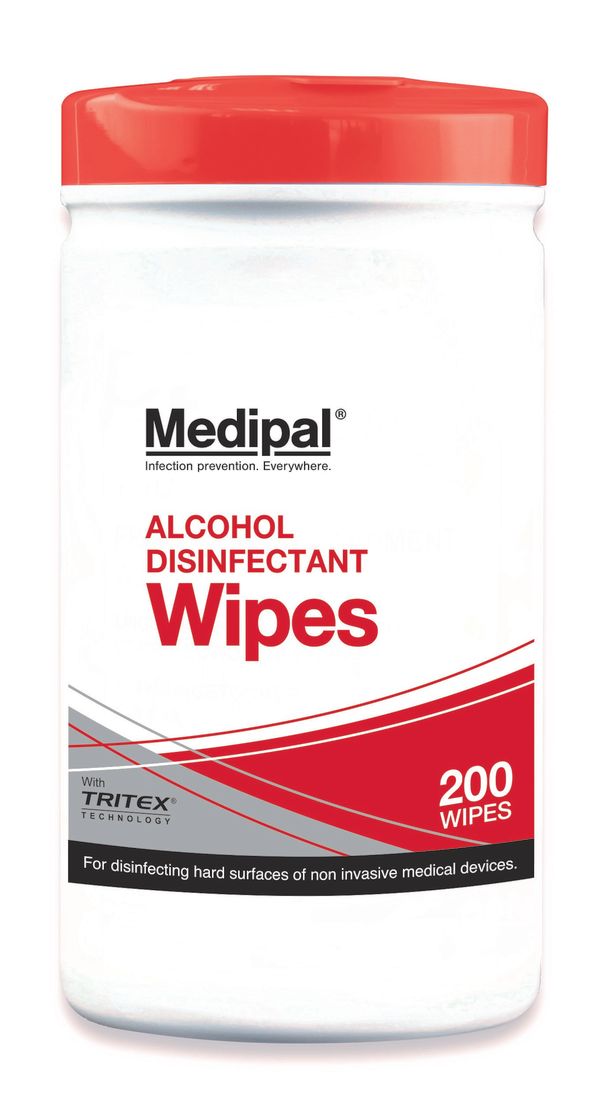
Alcohol Free Medipal Wipes
Alcohol Free Medipal Wipes
Add to Favorites
£5.50
£4.50
/ month
excl VAT
Add to FavoritesRemove from Favourites
Alcohol Free Medipal Wipes
Add to Favorites
£5.50
excl VAT
£210.13
excl VAT
£5.50
excl VAT
Best Seller
£45.00
excl VAT
Best Seller
£5.00 – £12.00
excl VAT
Best Seller
Add to FavoritesRemove from Favourites
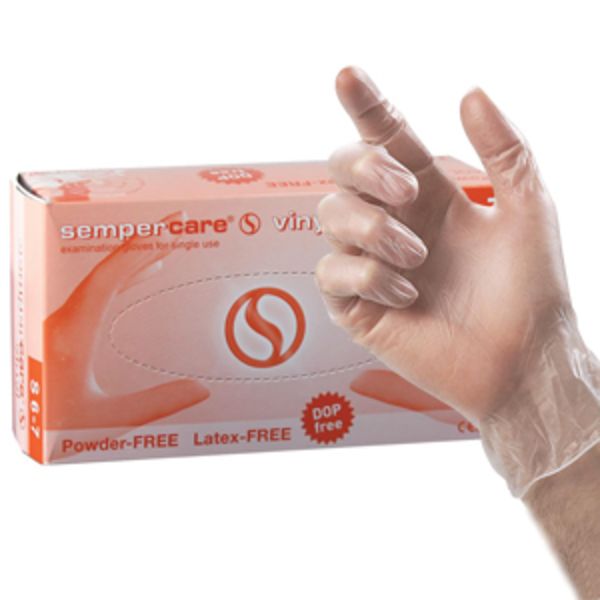
Williams Medical
Powder Free Vinyl Latex Free Gloves
Add to Favorites
£12.00
excl VAT
Best Seller
£16.00
excl VAT
£30.00
excl VAT